3D-printed Lithium-ion Battery Could Power Electric Vehicles, Drones
3D-printed Lithium-ion Battery Could Power Electric Vehicles, Drones

We rely on lithium-ion batteries every day to charge our smartphones, laptops, and many other electronics. One day they could power our electric vehicles. But the energy-storage capacity of lithium-ion batteries has struggled to keep up with the surging demands for their use.
Now, engineers at Carnegie Mellon University say they’ve found a way to significantly extend lithium-ion battery life by using a new method to print 3-D electrodes.
The lattice printing 3-D method could not only extend battery life, it could serve to create batteries made from materials like silicon, which would give the batteries faster recharging times and, when used in electric cars, longer range time. The low-weight and high-energy-capacity batteries the printing method create could also power small, light devices, like drones.
For You: Making the Next-Generation Lithium-Ion Batteries Safer, Longer-Lasting
All batteries contain two metal electrodes, a negatively charged anode and the positively charged cathode, separated by a substance called the electrolyte. A lithium-ion battery, or Li-ion battery, is a rechargeable battery in which lithium ions move from the negative electrode to the positive electrode during discharge, and then move back when charging
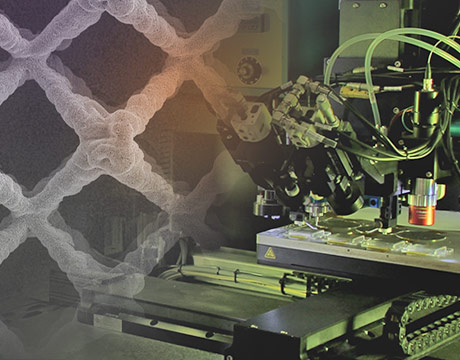
The Carnegie Mellon team’s electrodes are printed using Aerosol Jet technology, which assembles droplets one-by-one to create electrodes with lattice-like, interlaced structures with complex geometries that can be created using the current electrode-printing methods, said Rahul Panat, associate professor of mechanical engineering at the university.
Right now, lithium-ion battery electrodes are 3-D printed through an extrusion method that lays down “fingers” of the material one atop the other to form a solid block of material. Lithium has a hard time penetrating that solid block to charge the electrode, Panat said.
“Lithium has to penetrate throughout volume of electrode for it to be fully utilized,” he said. “In today’s commercially available batteries, you have about 30 to 50 percent of the lithium used.”
The chemical, on the other hand, can easily diffused throughout the channels and pores of the latticed electrode to completely saturate the electrode. The researchers have found that lithium saturates 100 percent of the battery’s electrode when it’s printed using their technique.
A 3-D printed, latticed electrode can make for a smaller battery that still carries the same charging capability of its larger, solid-electrode counterpart.
Prof. Rahul Panat, Carnegie Mellon University
Panut worked with Jonghyun Park, an assistant professor of aerospace engineering at Missouri University of Science and Technology to develop the new printing method.
Because it has more energy storage capacity, the latticed electrode can be used to make a smaller battery that still carries the same charging capability of its larger, solid-electrode counterpart.
“Or it could make the same-size battery that would hold much more charge,” Panat said.
Another benefit of the new process is that electrodes could now be created from widely available materials like silicone and oxide, which can store five to ten times more energy than the graphite lithium-ion batteries used today, Panat said. But that poses its own set of problems.
Because silicon can hold 10 times more lithium atoms, it expands significantly when charged.
“In the very first charging cycle, the silicon-electrode battery inside the test vehicle would expand to three times its original volume and crack. The battery would be dead,” Panat said. “The very fact these materials can hold more lithium becomes the reason for their cracking. It’s ironic. So stress relief is extremely important.”
To overcome that, the team added channels and pillars to the electrodes, which keep the battery from expanding. The lithium saturates the electrode, so it has no need to expand.
The new electrode-printing method could be commercialized in about four years, Panat said.
“Right now, we’re evaluating different materials, different use conditions, and looking at how many recharge cycles these batteries would work for,” he said.
Jean Thilmany is a freelance writer in St. Paul who frequently writes on engineering topics.
Read More: Battery CapacityGets a Boost 4D Printing Advances Additive Manufacturing Building Better Batteries



